The following text serves as a temporary placeholder, prior to expert moderation
ECoG
Moderated by: TBD
An Electrocorticogram (ECoG) can be seen as an in-situ EEG recording and allows for intra-operative detection of electrical activity in response to specific functional tasks. As such, it serves as a passive functional mapping alternative to the previously discussed ESM (1–3).
Technical Parameters
ECoG presents with much better spatial resolution (<1 cm) and comparable temporal resolution (milliseconds) as compared to EEG, as it does not require the signal to first pass through the skull, avoiding significant signal attenuation. Dependent on the sampling frequency, ECoG has the potential to sample in the millisecond range, enough to monitor the temporal dynamics of Local Field Potentials (LFPs).
Biological Substrate
ECoG is used in the context of epilepsy surgery as well as awake craniotomy surgery (4,5).
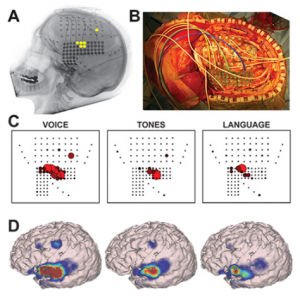
Using ECoG during awake craniotomy surgery, the surgeon aims to capture LFPs measured directly from the tissue. In particular, the broadband gamma range (>60 Hz) responses are of particular interest in this context (4), as they are highly task-specific (6) and thought to represent the average neuronal firing rate directly under the electrode (7–9). Literature also reports efforts to visualize cortical tumors themselves using ECoG-changes related to the presence of a tumor (2). In some cases, the ECoG grid itself is used not (only) for recording but for actual stimulation-based mapping, creating opportunity for a more standardized version of ESM. Here, the spatial resolution is again dependent on the interelectrode distance which is often less than a centimeter (10) (see also figures in the ESM chapter).
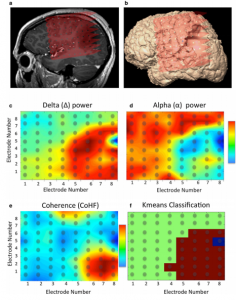
In the context of epilepsy surgery, ECoG is used to 1) identify the epileptogenic zone (with higher precision than pre-operative EEG could do), 2) to monitor changes in epileptiform activity during the resection and 3) to map out functionalities to avoid post-operative neurological deficits (11). The difficulty here is that intra-operative ECoG sampling is always limited in its time-window, often not allowing for the possibility to detect an actual seizure, but only interictal periods. Additionally, intra-operative ECoG can be complicated by the use of anesthesia. Some institutions therefore opt to place subdural or extraoperative ECoGs for a duration prior to the actual resection, to better map out the seizure onset zone, which can only be determined once an actual seizure is recorded. These subdural ECoG placements can be placed over a week prior to the actual resection to ensure seizure detection (11).
Intra-operative applicability
Using ECoG in an intra-operative awake craniotomy setting as a passive alternative to ESM has the great benefit of eliminating the risk of seizure elicitation. As compared to EEG, the direct cortical registration allows for more spatial resolution and sensitivity in detecting the epileptogenic zone. However, the field of view of intra-operative ECoG at the same time is also limited to the tissue under the craniotomy only. This means that intra-operative ECoG cannot replace the pre-operative whole brain work-up necessary to determine the operability of an epileptic condition (11). Additionally, ECoG does not allow for identification or delineation of involvement of deeper brain structures. In general, however, the technique is very suitable in an intra-operative setting given its ease of use, its flexibility as both a recoding and stimulating technique and its direct feedback. The technique seems especially valuable when combined with the neuro-navigation software during the mapping procedure, so that functional areas can be visualized respective to the structural MRI (Figure 1 and 2).
References
- Jeremy Hill N, Gupta D, Brunner P, Gunduz A, Adamo MA, Ritaccio A, et al. Recording human electrocorticographic (ECoG) signals for neuroscientific research and real-time functional cortical mapping. J Vis Exp. 2012;
- Boussen S, Velly L, Benar C, Metellus P, Bruder N, Trébuchon A. In Vivo Tumour Mapping Using Electrocorticography Alterations During Awake Brain Surgery: A Pilot Study. Brain Topogr [Internet]. 2016;29(5):766–82.
- Taplin AM, de Pesters A, Brunner P, Hermes D, Dalfino JC, Adamo MA, et al. Intraoperative mapping of expressive language cortex using passive real-time electrocorticography. Epilepsy Behav Case Reports. 2016;5:46–51.
- Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis: II. Event-related synchronization in the gamma band. Brain. 1998;
- Yang T, Hakimian S, Schwartz TH. Intraoperative electroCorticoGraphy (ECog): Indications, techniques, and utility in epilepsy surgery. Epileptic Disord. 2014;
- Miller KJ, Honey CJ, Hermes D, Rao RPN, denNijs M, Ojemann JG. Broadband changes in the cortical surface potential track activation of functionally diverse neuronal populations. NeuroImage. 2014.
- Ray S, Maunsell JHR. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol. 2011;
- Miller KJ, Sorensen LB, Ojemann JG, Den Nijs M. Power-law scaling in the brain surface electric potential. PLoS Comput Biol. 2009;
- Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci. 2009;
- Ritaccio AL, Brunner P, Schalk G. Electrical stimulation mapping of the brain: Basic principles and emerging alternatives. Journal of Clinical Neurophysiology. 2018.
- Kuruvilla A, Flink R. Intraoperative electrocorticography in epilepsy surgery: Useful or not? Seizure. 2003;12(8):577–84.
